Four out of more than 20,000 patients who took Pfizer’s trial vaccine for COVID-19 developed bell’s palsy.
In December 2020, the U.S. Food and Drug Administration (FDA) released additional data concerning a COVID-19 vaccine from the pharmaceutical company Pfizer ahead of a meeting with an independent panel of scientists and public health officials to discuss the new drug’s approval. Although the report showed that the vaccine was largely effective, some social media users singled out one seemingly scary statistic: Four of the patients who received the vaccine developed Bell’s Palsy, a type of temporary facial paralysis.
At this time there is no actual proof that the vaccine may have caused this, but 4 out of 20,000 is still one too many of a side effect that can alter my physical appearance. You can read the Vaccines and Related Biological Products Advisory Committee Meeting documents here.
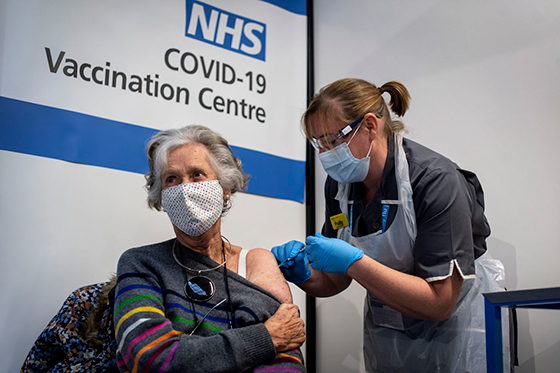
Doreen Brown, 85, receives the first of two Pfizer/BioNTech COVID-19 vaccine jabs administered at Guy’s Hospital in London, on Tuesday. U.K. health authorities gave emergency approval to the shots last week.
An F.D.A. advisory panel voted in favor of Pfizer’s vaccine, clearing one of the final hurdles before the agency authorizes the drug. It is likely to do so within days.
The F.D.A.’s vaccine advisory panel, composed of independent scientific experts, infectious disease doctors and statisticians, voted 17 to 4, with one member abstaining, in favor of emergency authorization for people 16 and older. With rare exceptions, the F.D.A. follows the advice of its advisory panels.
With this move, the United States may finally begin to slow the spread of the virus just as infections and deaths surge, reaching a record of more than 3,000 daily deaths on Wednesday. The F.D.A. is expected to grant an emergency use authorization on Saturday, according to people familiar with the agency’s planning, though they cautioned that last-minute legal or bureaucratic requirements could push the announcement to Sunday or later.
The initial shipment of 6.4 million doses will leave warehouses within 24 hours of being cleared by the F.D.A., according to federal officials. About half of those doses will be sent across the country, and the other half will be reserved for the initial recipients to receive their second dose about three weeks later.
sourced: NY Times
[ajax_load_more loading_style=”default” post_type=”post” scroll=”false”]